Abstract
Recent large-scale genetic screening of human blood cells have identified that somatic mutations associated with clonal expansions commonly arise with aging, even in the absence of cytopenias, myelodysplasia, or leukemia. Loss of function or dominant negative mutations in genes encoding epigenetic modifier enzymes such as DNMT3, TET2, and ASXL1 are most common in this "clonal hematopoiesis of indeterminate potential (CHIP)". However, a causal relationship between these mutations and clonal expansions or progression to myelodysplasia or leukemia is unclear, as it has been difficult to extrapolate from murine models due to the differences in lifespan, aging phenotypes and hematopoietic cell transformation susceptibility. We utilized CRISPR/Cas9 gene editing of hematopoietic stem and progenitor cells (HSPC) in the rhesus macaque model to study the relationship between mutations in these genes, and clonal expansion and progression to abnormal hematopoiesis.
We optimized CRISPR/Cas9 gene-editing strategies to introduce loss-of-function mutations in rhesus macaque CD34+ HSPC and applied this methodology to macaque autologous HSPC transplantation. We initially focused on the DNMT3A gene, which has been reported as most frequently mutated in CHIP. Macaque CD34+ HSPC were split into three equal fractions, transduced with one of three lentiviral vectors carrying either a gRNA for the control AAVS1 locus, DNMT3A exon 3, or DNMT3 exon 19 (the location of the "hotspot" putative dominant negative mutation in CHIP), and then electroporated with Cas9 mRNA. These edited cells were then transplanted into the autologous macaque following ablative total body irradiation. A second macaque also received autologous CD34+ cells electroporated with either AAVS1- or DNMT3A-targeting Cas9 ribonucleoprotein (RNP) complexes. Both animals engrafted promptly, with detection of low level (0-4%) indels at the on-target sites for AAVS1 and DNMT3A for up to 12 months post transplantation, but so far, we have not detected clonal expansion of DNMT3A-mutated HSPC in these two animals.
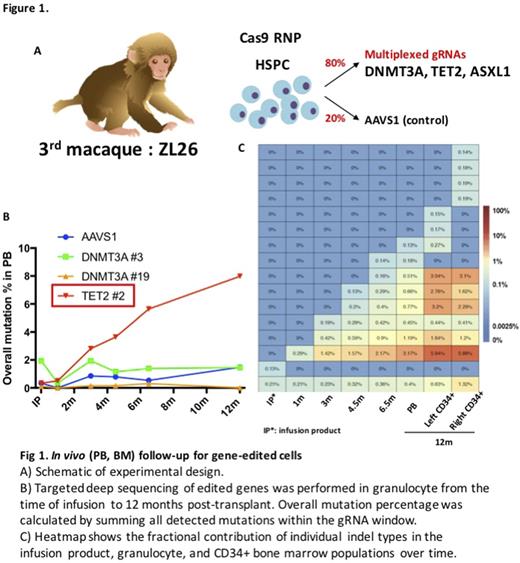
We next delivered Cas9 and a gRNA pool targeting DNMT3A, TET2, and ASXL1 (the top three mutated and expanded genes in CHIP) (80%) or AAVS1 (20%) into CD34+ HSPC, followed by autologous transplantation (Fig 1A). After engraftment, the level of indels at each target site in granulocytes was 2% or less. However, during the 12 months post-transplant, there was a gradual and marked expansion of the granulocyte fraction containing indels predicted to knock out TET2 function (Fig 1B). Of the 17 different indel signatures identified at 12 months, only two were over the limit of detection in the infusion product or at 1 month post-transplant (Fig 1C), suggesting that the expanding TET2 mutant clones originated from long-term HSPC rather than short-term progenitors or that rare, initially mutated clones were sufficient to initiate clonal expansions. Finally, TET2 mutation frequency in bone marrow CD34+ cells was more than 2-fold higher (~20%) than in mature granulocytes (8%) at 12 months post-transplant (Fig 1C), suggesting a possible block in differentiation or a defect in release from the marrow for TET2 mutated cells.
In conclusion, we have successfully created a non-human primate model of CHIP utilizing autologous transplantation of CRISPR/Cas9-edited HSPC, demonstrating gradual expansion of TET2-mutated clones. In contrast, simple indel formation at the DNMT3A locus was not sufficient to result in clonal expansion in the three animals studied to date, and a strategy utilizing HDR to specifically knock-in the hotspot base pair recurrently mutated in CHIP will be required to further investigate the relationship of DNMT3A to CHIP. Taken together, these approaches should improve our understanding of the effects of TET2 loss of function on the development of CHIP and its link to hematologic and cardiovascular disease, as well as provide a model for the testing of preventative interventions for clonal expansion and progression to frank malignancy.
Dunbar: Novartis/GSK to institute: Research Funding. Winkler: Novartis/GSK to institute: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.